Steroid Hormones Act Through a Conserved Family of Intracellular/nuclear Receptor Proteins
The steroid hormone receptors (SHRs) vest to the nuclear receptor (NR) gene superfamily, which represents a class of ligand-dependent intracellular transcription factors that modulate factor expression in response to lipophilic ligands [1,2]. These receptors play diverse roles in cell differentiation/development, proliferation, and metabolism and are associated with numerous pathologies such as cancer, cardiovascular affliction, inflammation, and reproductive abnormalities. Thus, SHRs are broadly implicated in normal physiological development and metabolism and represent therapeutic targets for a wide range of human diseases [3-5]. All the SHRs have a central DNA binding domain (DBD) and a carboxy-last ligand-binding domain (LBD) with conserved tertiary structures (Figure 1A). The SHRs too contain an amino-terminal domain (NTD), which is divergent amongst NR family members. Within the NTD of SHRs lies a powerful activation office, AF1 region, which can activate transcription in a ligand contained manner. The LBD contains a 2d activation office, AF2 that maps to a surface-exposed hydrophobic pocket, providing a docking site for coregulatory proteins [6]. Ligand binding to its receptor results in transactivation of specific target genes in a tissue/prison cell- and promoter- specific manner.
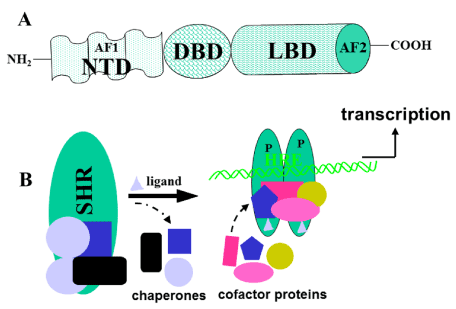
Figure i. A) A general topology of the SHRs, showing 3 principal functional domains. The DBD is the near conserved domain and consists of 2 zinc fingers. Information technology is responsible for site-specific Deoxyribonucleic acid binding at its response chemical element. The second well-nigh conserved domain, LBD, is responsible for ligand binding, interaction with heat shock proteins to stabilize receptor, homo and/or hetero-dimerization, and contains a ligand-dependent transcriptional activation region (AF2). Among the members of superfamily, the North-last domain (NTD) is the least conserved, both in size and primary sequence, and AF1, lies within this domain. This region is rich in acidic amino acids, and appears to be mostly unstructured. B) Model for the general mechanism of activity of SHRs. Without ligand, the receptor (SHR) is present in an inactive complex associated with heat shock and other chaperone proteins. Ligand (gray triangle) binding releases these proteins, hyper-phosphorylates (P) and receptor adopts a conformation that binds with high affinity to its specific DNA response chemical element (HRE) and diverse cofactor proteins, to regulate transcription. The protein bounden partners are shown by unlike colors and shapes.
Ligands allosterically control the interactions of SHRs with coactivators and corepressors by influencing the conformation of a brusk helix, referred to every bit Helix 12 or AF2 (activation function 2), towards the carboxy-terminal end of the LBD. Coactivators bind to the AF2 surface via the amino acid motif LxxLL, in which the Leucine residues dock into the hydrophobic crack [seven,8]. Binding specificity is added by oppositely charged amino acids at either end of the NR hydrophobic cleft that form a charge clamp with the LxxLL peptide backbone. The mechanism of transcriptional activation of SHRs past AF2 is via recruitment of these coactivators, which mediate chromatin remodeling and recruit the basal transcription apparatus. On the other mitt, bounden of an adversary to the ligand bounden pocket prevents coactivator recruitment to the AF2 via the LxxLL motif. Thus, AF2 provides allosteric control of transcription mediated past its dynamic localization. AF2 is the structural link between a ligand-bound SHR and coactivator, just the details of this communication and its differential regulation by receptor accept remained elusive. Several contempo structural and functional studies are beginning to shed light on the details of these signaling pathways, facilitating an improved understanding of how small molecule steroid receptor modulators (SRMs) control cofactor recruitment [nine-11].
According to the classic mechanism of steroid action a ligand-bound SHR enters the nucleus and binds to its response element DNA site (Figure 1B). This bounden allows germination of a transcriptional initiation circuitous, directly past interactions between AF1 and AF2 and the complex, or indirectly through co-activators. Our understanding of how AF2 functions has been greatly enhanced by the delineation of the basic LBD construction [12,thirteen]. Clinically this phenomenon has extensively been exploited for the development of small-scale molecule SHR modulators (SRMs). Nonetheless, about of these SRMs showroom fractional agonist or mixed agonist activities that may non exist desirable. This undesired effects of SRMs have often been attributed to their current inability to restrict SHR actions to specific organ/factor targets. Though, it has been suggested that the tissue-specific residual activity of SHRs in the presence of SRMs may be mediated via AF1, yet therapeutic targeting of the AF1 has been limited [12]. A major unresolved consequence for SHR signaling and transcriptional activities is the nature of cantankerous talk between AF1 and AF2, particularly every bit modulated by SRMs, DNA and other interacting protein partners. This is despite the fact that full receptor activity requires a synergistic effects of both AF1 and AF2. Though it is however not well established whether this association of the AF1 and AF2 is always direct, indirect, or both (Figure 2).
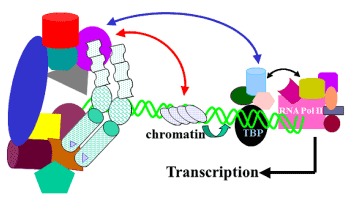
Figure 2. A model for the regulation of transcription by NHR cofactor assemblies. Both AF1 and AF2 regions recruit certain specific cofactors. A bridge is formed betwixt AF1 and AF2 through these and/or other cofactor(s). AF1 and AF2 can also interact directly. Bridging the cofactor(south) are largely regulated by prison cell-blazon and ligand. The circuitous alters local chromatin construction (red arrow), catalyzes histone acetylation or deacetylation, and affects the stabilization of the transcription pre-initiation complex. The receptor complex, jump to DNA enhancer sites, thus recruits and regulates Pol Two via direct associations with specific subunits of the mediator complex (blue arrow), which makes a span between the receptor and Pol Two. The activity of kinases and phosphatases regulating signaling pathways too contribute to this process by altering the country of phosphorylation of both receptor and cofactors (not shown). The receptor:cofactor assembly may also interact straight with the basal transcription machinery at TBP/TATA box to regulate transcription.
Because SHRs regulate many genes in many tissues, synthetic SRMs ordinarily show beneficial therapeutic furnishings and unwanted side effects that limit their clinical uses. Major goals in the therapeutic targeting of SHRs therefore include attaining a ameliorate understanding of the mechanisms underlying their actions in specific cell types and ways in which to selectively modulate their activities [fourteen]. Therefore, importance of SHRs in the regulation of function of all major organ systems demands a total understanding of their mechanism of activity. Meliorate agreement SHRs' inter-domain connectivity should provide the basis for answering several outstanding issues such as the nature of the structural shifts that occur in the SHR upon binding ligand and/or the correct DNA sites in the rapidly reversible interactions betwixt receptor and other proteins, chromatin and DNA sites, and how sure ligands human action as agonists in some tissues and antagonists or very weak partial agonists in others. Information technology is predictable that SHR construction information will ultimately help explain how diverse SRMs and other ligand contained signaling mechanisms are able to elicit selective biological effects, via interactions and/or communication betwixt the AF1, AF2 in diverse tissues. Though the 3-D structures of the independently expressed recombinant DBD and LBD of many SHRs are known, as yet the full structure of none of the member proteins is known. As for the NTD structure is concerned, we are only start to empathise and at that place is no iii-D construction of NTD bachelor.
Contempo studies take shown that the SHRs' NTD/AF1 exist every bit an ensemble of conformers also known as "intrinsically disordered (ID)", which collectively announced to be unstructured [15-17]. This dynamic unstructured nature of SHR NTDs has been problematic in preventing crystallization and high-resolution diminutive structures. Importantly, this problem is not unique to SHRs, and many activation domains of transcription factors contain ID regions that have been refractory to crystallization [xviii,nineteen]. In recent years, it has become quite evident that these regions of disorder in signaling proteins seem to promote molecular recognition through their ability to form surfaces capable of binding specific binding partners [xx]. These dynamic ensembles of interconverting conformers of SHRs' ID AF1/NTD are capable of undergoing a disorder-to-guild transition upon interaction with macromolecules including other proteins or DNA [18,19]. This structural flexibility and process of "coupled folding and binding" appears to have certain advantages for intra- and intermolecular interactions as compared with ordered structural motifs. It has been suggested that the tissue-specific residual activity of SRM-bound SHRs may mainly be mediated via AF1 and that the relative functional importance of AF1 may exist decided by specific SRM-induced conformational changes in either LBD or transmitted allosterically to the NTD [12,14]. It therefore makes sense to investigate the possibility of identifying receptor modulators that act to regulate AF1 activity, which could complement or supplant existing SRMs. However, the therapeutic targeting of ID NTD/AF1 using small-molecule inhibitors of receptor function has been limited despite the critical role that its structural flexibility tin play in allosteric modulation of synergy between AF1 and AF2.
Targeting ID proteins by small molecules to block protein-protein interactions is a chop-chop evolving field, and therefore identifying compounds that bind to NTD/AF1 could be promising minor molecules for SHR-based therapeutics. A major challenge, though, with developing compounds that target ID NTD of SHRs is the express noesis of the physiologically relevant NTD/AF1-interacting coregulatory protein(s) capable of inducing functionally active folded state of NTD/AF1. Meaningful screens for drugs that block interactions of proteins with NTD/AF1s will therefore require identification of the most functionally important coregulatory proteins or poly peptide complexes that interact with the NTD/AF1. Notwithstanding, a multifactorial approach of SRMs, coregulators, and assorted pocket-size-molecule inhibitors in an organ-dependent context may provide the additional selectivity needed to target selected genes and thereby reduce the number of undesirable side effects in current endocrine-related therapies.
- Chambon P (1996) A decade of molecular biological science of retinoic acrid receptors. FASEB J x: 940-954. [Crossref]
- Mangelsdorf DJ, Evans RM (1995) The RXR heterodimers and orphan receptors. Cell 83: 841-850. [Crossref]
- McKenna NJ, O'Malley BW (2002) Combinatorial control of gene expression past nuclear receptors and coregulators. Cell 108: 465-474. [Crossref]
- Kumar R, Thompson EB (2012) Folding of the glucocorticoid receptor North-terminal transactivation part: dynamics and regulation. Mol Prison cell Endocrinol 348: 450-456. [Crossref]
- Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ (2001) Nuclear receptors and lipid physiology: opening the Ten-files. Science 294: 1866-1870. [Crossref]
- Huang HJ, Norris JD, McDonnell DP (2002) Identification of a negative regulatory surface within estrogen receptor alpha provides show in back up of a office for corepressors in regulating cellular responses to agonists and antagonists. Mol Endocrinol 16: 1778-1792. [Crossref]
- Shiau AK, Barstad D, Loria PM, Cheng 50, Kushner PJ, (1998) The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Prison cell 95: 927-937.
- Darimont BD, Wagner RL, Apriletti JW, Stallcup MR, Kushner PJ, et al. (1998) Structure and specificity of nuclear receptor-coactivator interactions. Genes Dev 12: 3343-3356. [Crossref]
- Johnson AB, O'Malley BW (2012) Steroid receptor coactivators 1, ii, and 3: critical regulators of nuclear receptor action and steroid receptor modulator (SRM)-based cancer therapy. Mol Cell Endocrinol 348: 430-439. [Crossref]
- Nilsson Southward, Koehler KF, Gustafsson JÅ (2011) Evolution of subtype-selective oestrogen receptor-based therapeutics. Nat Rev Drug Discov ten: 778-792. [Crossref]
- McDonnell DP, Wardell SE (2010) The molecular mechanisms underlying the pharmacological actions of ER modulators: implications fornew drug discovery in chest cancer. Curr Opin Pharmacol 10: 620–628. [Crossref]
- Kumar R, McEwan IJ (2012) Allosteric modulators of steroid hormone receptors: structural dynamics and gene regulation. Endocr Rev 33: 271-299. [Crossref]
- Brzozowski AM, State highway AC, Dauter Z, Hubbard RE, Bonn T, et al. (1997) Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 389: 753-758. [Crossref]
- Simons SS Jr, Edwards DP, Kumar R (2014) Minireview: dynamic structures of nuclear hormone receptors: new promises and challenges. Mol Endocrinol 28: 173-182. [Crossref]
- Goswami D, Pascal B, Kumar R, Edwards DP, Griffin PR (2014) Structural dynamics and inter domain crosstalk of PR-TBP interaction probed by hydrogen/deuterium exchange Mass Spectrometry. Structure 22: 961-973. [Crossref]
- Kumar R, Moure CM, Khan SH, Callaway C, Grimm S, et al. (2013) Regulation of the structurally dynamic disordered amino-terminal domain of progesterone receptor by protein induced folding. J Biol Chem 288: 30285-30299. [Crossref]
- Khan SH, Awasthi S, Guo C, Goswami D, Ling J, et al. (2012) Bounden of the amino concluding region of coactivator TIF2 to the intrinsically disordered AF1 domain of the glucocorticoid receptor is accompanied by conformational reorganizations. J Biol Chem 287: 44546-44560. [Crossref]
- Liu J, Perumal NB, Oldfield CJ, Su EW, Uversky VN, et al. (2006) Intrinsic disorder in transcription factors. Biochemistry 45: 6873-6888. [Crossref]
- Dunker AK, Uversky VN (2010) Drugs for 'poly peptide clouds': targeting intrinsically disordered transcription factors. Curr Opin Pharmacol 10: 782-788. [Crossref]
- Ferreon Air-conditioning, Ferreon JC, Wright PE, Deniz AA (2013) Modulation of allostery past protein intrinsic disorder. Nature 498: 390-394. [Crossref]
2021 Copyright OAT. All rights reserv
Editorial Information
Editor-in-Chief
Hieronim Jakubowski
Rutgers Academy-New Jersey Medical School
Article Type
Editorial
Publication history
Received date: November 24, 2016
Accepted date: December 14, 2016
Published date: December xvi, 2016
Copyright
© 2016 Kumar R. This is an open-admission article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in whatsoever medium, provided the original author and source are credited.
Citation
Kumar R (2016) Gene regulation by the steroid hormone receptors: new promises and challenges for therapeutic targeting. Biomed Genet Genomics1: DOI: ten.15761/BGG.1000121.
Corresponding writer
Raj Kumar
Department of Bones Sciences, The Commonwealth Medical Higher, 525 Pine Street, Scranton, PA-18509, United states of america Tel: 570-504-9675; Fax: +570-504-9660

Figure 1. A) A full general topology of the SHRs, showing iii primary functional domains. The DBD is the most conserved domain and consists of two zinc fingers. It is responsible for site-specific DNA binding at its response element. The second most conserved domain, LBD, is responsible for ligand binding, interaction with heat shock proteins to stabilize receptor, homo and/or hetero-dimerization, and contains a ligand-dependent transcriptional activation region (AF2). Amidst the members of superfamily, the North-terminal domain (NTD) is the least conserved, both in size and chief sequence, and AF1, lies inside this domain. This region is rich in acidic amino acids, and appears to exist mostly unstructured. B) Model for the full general mechanism of action of SHRs. Without ligand, the receptor (SHR) is nowadays in an inactive complex associated with heat stupor and other chaperone proteins. Ligand (grey triangle) binding releases these proteins, hyper-phosphorylates (P) and receptor adopts a conformation that binds with high analogousness to its specific Deoxyribonucleic acid response chemical element (HRE) and various cofactor proteins, to regulate transcription. The protein binding partners are shown by different colors and shapes.

Figure 2. A model for the regulation of transcription by NHR cofactor assemblies. Both AF1 and AF2 regions recruit certain specific cofactors. A bridge is formed between AF1 and AF2 through these and/or other cofactor(s). AF1 and AF2 can too collaborate directly. Bridging the cofactor(due south) are largely regulated by cell-type and ligand. The complex alters local chromatin construction (red arrow), catalyzes histone acetylation or deacetylation, and affects the stabilization of the transcription pre-initiation complex. The receptor complex, bound to Dna enhancer sites, thus recruits and regulates Politician II via directly associations with specific subunits of the mediator complex (blue arrow), which makes a bridge between the receptor and Pol Two. The activity of kinases and phosphatases regulating signaling pathways besides contribute to this process by altering the land of phosphorylation of both receptor and cofactors (non shown). The receptor:cofactor assembly may likewise interact directly with the basal transcription machinery at TBP/TATA box to regulate transcription.
Source: https://www.oatext.com/Gene-regulation-by-the-steroid-hormone-receptors-new-promises-and-challenges-for-therapeutic-targeting.php
0 Response to "Steroid Hormones Act Through a Conserved Family of Intracellular/nuclear Receptor Proteins"
Post a Comment